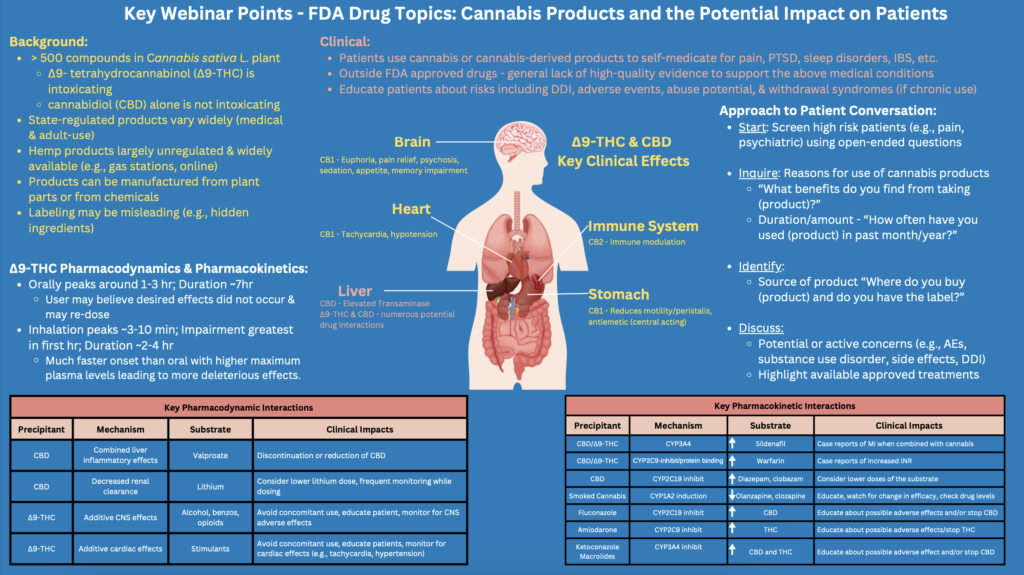
Federal officials from the U.S. Food and Drug Administration (FDA) met this week for a wide-ranging educational webinar about cannabis, outlining matters such as the plant’s chemical makeup, differences between marijuana and hemp, clinical and therapeutic effects of cannabinoids as well as ongoing health and safety concerns around the drug’s use.
Hosted by FDA’s Division of Drug Information, which is part of the agency’s Center for Drug Evaluation and Research (CDER), the webinar was designed to provide information “targeting the needs of all health care professionals, including physicians, physician assistants, nurse practitioners, nurses, pharmacists, pharmacy technicians, and certified public health professionals.”
The event comes as the federal government considers the Biden administration’s proposed rescheduling of cannabis, which would move it from Schedule I to Schedule III of the federal Controlled Substances Act (CSA), effectively legalizing certain FDA-approved forms of medical marijuana.
Meanwhile, many U.S. doctors have for years expressed that they feel “completely in the dark” about marijuana’s medical benefits and risks despite state-level legalization and surging interest from both patients and adult consumers.
FDA’s cannabis-focused discussion on Tuesday, part of the agency’s ongoing Drug Topics series of educational webinars, focused predominantly on basic cannabis information, such as different species
Read full article on Marijuana Moment


