
Australians could purchase medicinal cannabis over the counter from 2021. This is according to the announcement made by the Therapeutic Goods Administration (TGA) – a division of the Australian Department of Health. The TGA announced they plan to make cannabidiol (CBD) products are available without a prescription.
June 2021
According to the TGA, the implementation date is set for June 2021. Cannabidiol is one of the major ingredients in cannabis, used for medicinal purposes. In Australia, CBD is currently listed as “prescription-only medicine.”
If implemented, the changes would give many Australians a relief, saving them a trip to the doctor. Patients would now be able to access CBD products over the counter after consulting a pharmacist. However, the TGA also stated the privilege would come with several limitations.
For instance, patients would only have a maximum daily dose of 60mg and a restriction of a 30-day supply. Additionally, the products would only be available for adults aged 18 years and above. They would be packed in a blister, strip, or container with a child-resistant closure.
Josh Fegan is the CEO of Althea – a licensed producer, exporter, and supplier of pharmaceutical-grade cannabis. He applauded this development, saying it was one of the biggest developments in the industry.
The interim decision reflects the significant shift in community and government attitudes towards medicinal cannabis since it was legalized in Australia in late 2016, which has seen it move from a fringe alternative towards an accepted mainstream option. We are excited by the TGA’s decision to down schedule CBD products and see this development as a big step forward for prescription cannabis products already available in Australia, Fegan said.
The government will make a final decision on the proposed changes in November.
Cannabis patient number increasing
This development comes at a time when the number of patients using medical cannabis is increasing. In August, the government approved about 5,270 patients for medical cannabis treatment through the SAS Category B pathway. This was the second consecutive month in which the country recorded more than 5000 individual approvals.
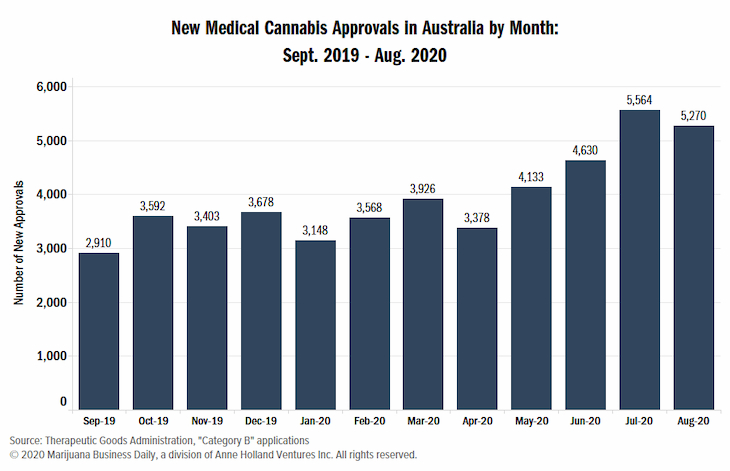
The August figure represented a 5% drop from the previous month. However, the figure is still 17 percent more than the average monthly approvals witnessed in the previous five months. This shows a positive trend, with most medical marijuana prescriptions in Australia being under the SAS B pathway.


